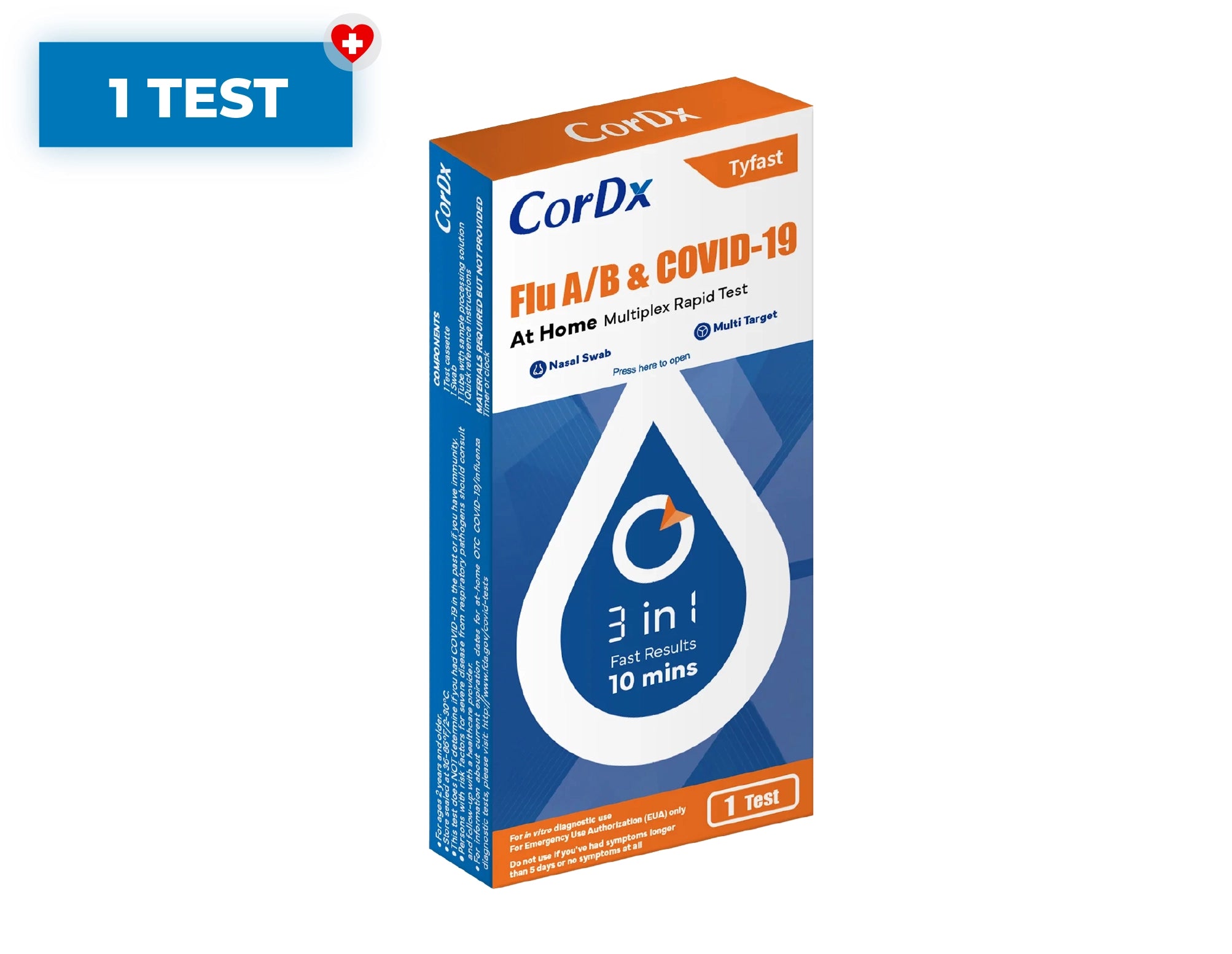
CorDx TyFast Flu A/B & COVID-19 At Home
This product is non-returnable, non-exchangeable, and will not be
refunded after your order has been processed.
CorDx Tyfast Flu A/B & COVID-19 At Home Multiplex Rapid Test
Authorized under Emergency Use Authorization only.
This product has not been FDA cleared or approved but has been authorized by FDA under an EUA.
Leverage the power of CorDx’s innovation with CorDx Tyfast Flu A/B &
COVID-19 At Home Multiplex Rapid Test. This leading-edge lateral flow assay
brings rapid, accurate, and simultaneous qualitative detection of influenza
A, influenza B, and SARS-CoV-2 antigens right to the at-home in 10 mins.
Designed for convenience and accuracy, this test is a game-changer for
individuals showing symptoms of respiratory infection.
-
Swift Clarity: Receive results rapidly in 10 minutes for early
decision-making. -
Trusted Precision: Clinically validated, providing confidence in
every test, simultaneously identifies influenza A, influenza B, and
SARS-CoV-2 antigens. -
User-Friendly: Easy to use design, effortless self-administration,
whether at home or on the go.
Click here
to access the quick reference instructions for use.
Kit Contents
Each test kit includes:
- Test cassette
- Tube with sample processing solution
- Sterile swab
- Quick reference instructions for use
Test Preparation and Usage
Preparing for the Test
- Read all the instructions before you start the test.
- Check the test’s expiration date (EXP). Do not use an expired test.
-
Wash your hands with soap and water for 20 seconds and dry them
thoroughly, or use hand sanitizer. - Use a flat level surface (such as a table or countertop) for testing.
- Use a timer during the test.
- Make sure you have all the test components before you begin.
-
Bring test kit to room temperature (59~86°F /15~30°C). Perform test at
room temperature. Testing under conditions other than room temperature may
lead to inaccurate results.
Performing the Test
-
STEP 1
Remove the swab from the pouch. Note: Be careful not to touch the
swab tip (soft end) with your hand. -
STEP 2
Insert the entire soft end of the swab into the nostril no more than
3/4 of an inch (1.5 cm). Firmly and slowly rotate the swab 5 times,
brushing against the inside walls of the nostril to ensure both mucus
and cells are collected. Do not push the swab further if you meet
resistance. For young children, do not insert more than 1/2 inch.
Using the same swab, repeat this process for the other nostril to
ensure an adequate sample is collected from both nostrils. -
STEP 3
Insert the swab into the tube until it touches the bottom. Rotate the
swab at least 10 times while pressing the swab head against the bottom
and side of the tube. -
STEP 4
Remove the swab while squeezing the sides of the tube. Attach the
dropper tip firmly onto the tube. -
STEP 5
Slowly squeeze the tube and dispense 3 drops of solution into the
sample well. Note: Invalid results can occur if less than 3 drops are
added to the sample well. Note: False results can occur if the test is
read before 10 minutes or after 30 minutes. -
STEP 6
Wait 10 minutes. Read the result after 10 minutes but before 30
minutes.
How to Interpret the Results
Invalid Results: If the control line (C) is not visible,
the test is invalid, even if any test line is visible. Re-test with a new
swab and new test device.
Negative Results: If the control line (C) is visible, but
no other lines appear, the test is negative. To increase the chance that the
negative result for COVID-19 is accurate, you should test again in 48 hours.
If you still have COVID-19, Flu B, Flu A, or symptoms, you should seek
follow-up care with your healthcare provider.
Positive Results: If the control line (C) is visible and
one or more lines appear(s) for any of the viruses, the test is positive for
that or those viruses.
Note: It is possible to have more than one positive test
line, which could indicate a co-infection with influenza A, B, and/or
SARS-CoV-2. If more than one positive test line is observed, retest with a
new sample and new test kit. If you continue to have a “dual positive”
result, you should contact your healthcare provider to be tested with a
molecular assay to confirm your results.